
While there are a wide range of scenarios in which functional echo may be useful, we would argue that the 3 key assessments are:
PDA
PPHN
Filling, Contractility and Flow
Despite multiple trials of treatment, there is still no consensus approach to prolonged patency of the ductus arteriosus. For a list of papers tackling this thorny issue see our Resources section.
But no matter what your view on how interventionist to be when it comes to ductal treatment, we would still argue that a comprehensive echo assessment should be central to any assessment.
The echo assessment has 2 key elements:
1 - Exclusion of structural heart disease. In particular coarctation of the aorta. If coarctation is present the duct will be patent to attempt to conserve lower body blood flow. Closing it would be disastrous. See the structural assessment page.
2 - Assessment of 'haemodynamic significance'. This is in itself a difficult area, but there is clearly a difference between small, tightly constricted PDAs (generally with continuous high velocity flow, forward diastolic flow in the descending aorta and normally sized left-sided chambers) and large non-restricted PDAs (generally with low velocity flow in diastole, reversed diastolic flow in the descending aorta and dilated left-sided chambers).
As ever, when performing an echo to assess ductal patency, one should always conduct a full structural assessment.
When the first 5 views have been achieved it's time to examine the duct. Using a high sagittal view, try to visualise the proximal pulmonary trunk and the distal aortic arch. The duct joins these two structures.
If large it can be seen on 2D imaging:
But colour Doppler is extremely useful:
The PDA shown above appears to be very large. Some other ducts are clearly tiny:
The difficulty with assessment comes with moderate ducts. Which of these two infants has the larger PDA?:
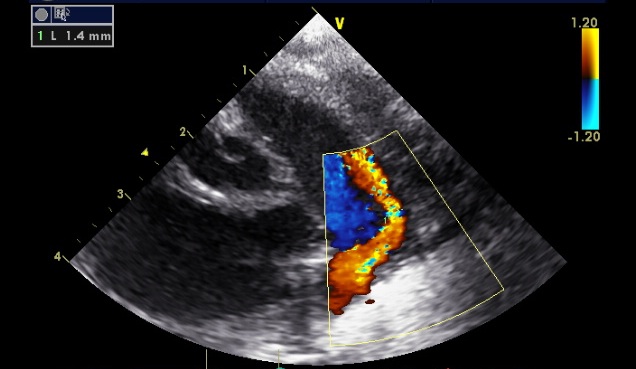
Of course it's a trick question. This is the same infant, with the same PDA. The two images were taken 20 seconds apart. The only difference between the two is the colour gain setting on the echo machine. By altering the gain the PDA can be made to appear to be either 1.4mm:
or 2.4mm:
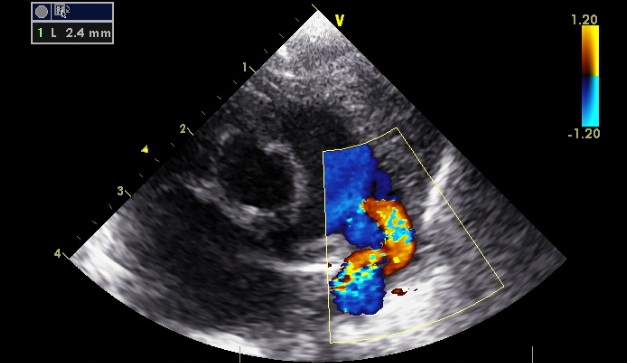
Standard advice is to adjust gain until flow cannot be seen outside blood vessels, but this decision is clearly arbitrary. To try to clarify which ducts are 'haemodynamiclly significant' one needs to examine other parameters:
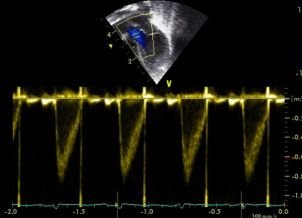
1 - Flow profile within the duct. A constricting PDA tends to have high velocity flow throughout the cardiac cycle:
A non-restrictive duct will have lower flow during cardiac diastole.
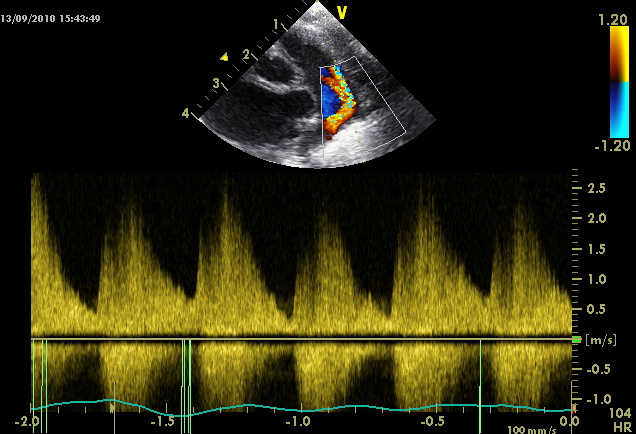
As a rule of thumb if the end-diastolic flow through the PDA is less than 50% of the peak systolic velocity this suggests poor constriction.
2 - Flow pattern in the descending aorta (DAo).
Our MRI assessments of shunt volume through the PDA suggested that descending aortic flow reversal is the most reliable marker of high volume PDA shunt. Read the full paper here.
Image the DAo at around the level of the diaphragm. The easiest way to do this is to tilt caudally from your standard sagittal ductal view, look for the vertebrae, and then tilt slightly to the left. Using pulsed wave Doppler and angle correction the flow profile can be seen. An infant with a constricted PDA will have forward diastolic flow in the DAo. An infant with a non-restrictive duct will have reversed diastolic flow in the DAo as blood which advanced forwards in systole is 'sucked' back up through the PDa and into the lungs in diastole:
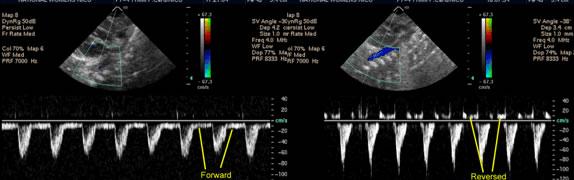
3 - Dilatation of left-sided cardiac chambers. High volume through the duct passes through the pulmonary vasculature and returns to the left atrium. Particularly with prolonged shunting the left atrium and even the left ventricle become dilated. Dilatation can be assessed by 'eyeballing' the difference between normally filled chambers and dilated chambers.
But can also be quantified by measuring the left atrium to aortic root ratio:
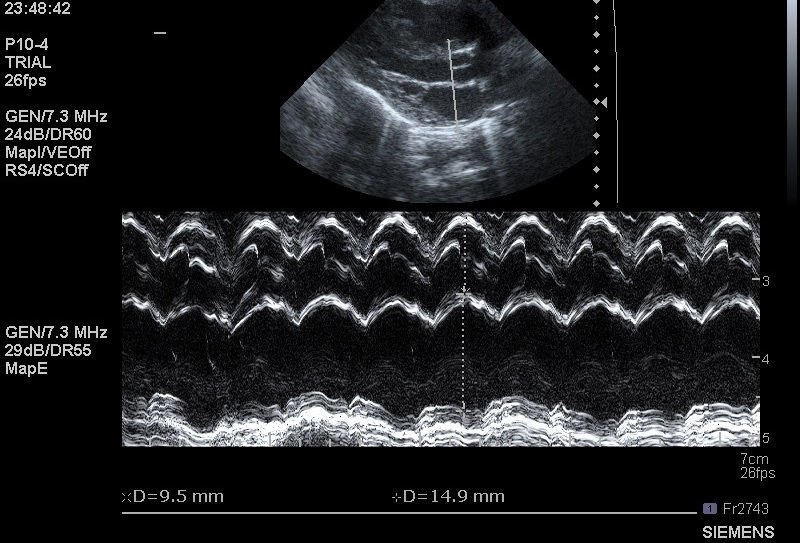
An LA:Ao > 1.4 suggests dilatation.
This is arguably one of the most immediately valuable uses of functional echo.
It is also the one where the risk of misdiagnosis is highest. Always perform a full structural assessment.
Differentiating PPHN and congenital heart disease in the middle of the night is not easy. At this point we refer you again to this Disclaimer. If you are in any doubt start Prostin, and get an urgent cardiology review.
Once structural heart disease has been excluded, assess the severity of PPHN. As ever with echo, there is no single measure that ensures the correct assessment is made. We would argue that an echo assessment of PPHN severity requires 3 key components:
1 - Direction of ductal shunt. After the first hour or two of life any PDA should have systemic-to-pulmonary shunting. This is generally visible as a jet of orange flow on the ductal view. However in PPHN the shunt is pulmonary-to-systemic, and therefore the ductal flow appears blue:
Keep this mental image in mind. it is ALWAYS abnormal. It is often referred to as the 'three-legged stool':
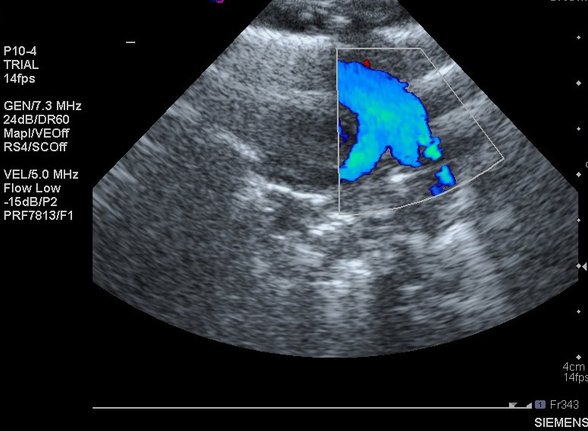
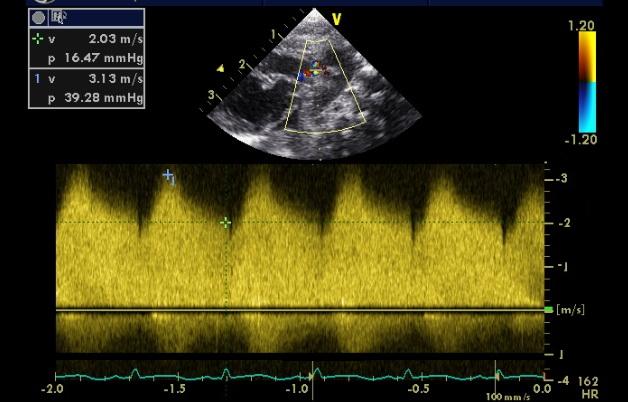
2 - Quantification of TR jet velocity. A simplified version of the Bernoulli equation suggests that the pressure gradient between two chambers across a narrow orifice is equal to 4 times the velocity squared. Since the tricuspid valve is closed during ventricular systole any regurgitant jet is occuring through a very narrow orifice:

By estimating the velocity of the jet (if present) the pressure gradient across the valve can be estimated as 4 times the velocity squared. For example a velocity of 2 m/s suggests a gradient of 16mmHg. A velocity of 4 m/s suggests a gradient of 64mmHg. Remember to add around 5mmHg (an estimate of right atrial pressure) to the gradient to calculate the pulmonary artery pressure, and to compare this pressure level to systemic systolic blood pressure (not the mean systemic blood pressure).
3 - Evidence of ventricular septal distortion. In the physiological situation the left ventricular pressure is higher than the left. Therefore the intraventricular septum will 'bow' into the right ventricle - producing the classic circular left ventricular shape (A, bleow). However in PPHN the right ventricular pressure is often equal to, or even above the left. Therefore the intraventricular septum will be flattened, or even bow into the left ventricle (B, below):
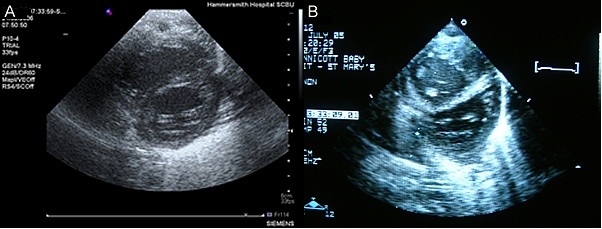
As well as these 3 components 2 other patterns are worthy of mention. The most severe, and a rare but important diagnostic trap, is functional pulmonary atresia. In these infants the pulmonary resistance is so high, and right ventricular function so poor, that the contracting right ventricle cannot open the pulmonary valve. So the long axis view looks like this:
This can obviously be mistaken for a structural atresia, resulting in unnecessary referral for a cardiology opinion, and insufficient focus on therapies to decrease pulmonary vascular resistance. The two clues that the atresia is functional rather than structural is that the valve leaflets here look thin and crisp, rather than the thickened valves seen in pulmonary atresia/stenosis:
In addition there is often regurgitation through the immobile pulmonary valve in PPHN, which clearly would not be the case in a structural atresia:
Before getting too carried away with echocardiographic assessments at the cotside, it is important to remember that all echo measures (and indeed all other measures!) are subject to measurement error. In neonatal functional echo, even in the most experienced hands, errors can be in the order of 30-40%.
We do not feel this means that measurement should not be performed. Rather we feel that measurements should be interpreted with caution, and measurements of multiple parameters should be taken to avoid excessive reliance on a single measure.
A few standard principles apply for ‘good housekeeping’. When making any functional take it from 3-5 consecutive cardiac cycles rather than a single cycle. This will help reduce measurement error and true variability related to the respiratory cycle. Ensure that the child is settled - while abnormal anatomy can be excluded even in the crying infant, abnormal function cannot. All flow measures depend on measuring vessel diameter (and thus calculating area from pi r squared) and velocity of flow from pulsed wave Doppler. For measures of diameter focus on making the measure as accurate as possible - any inaccuracy is amplified when the radius measure is squared to calculate the area. For the velocity measurement be sure to use pulsed wave Doppler, and place the range gate at the same point where diameter was calculated. Measuring velocity anywhere else (or using continuous wave Doppler which measures highest velocity signal anywhere along the imaging plane) will produce inaccuracies.
This measurement is robust and well validated. However in the newborn infant it is obviously confounded by ductal shunting.
Measure the aortic diameter from the parasternal long axis view.
Zoom in on the valve, and try to get the outflow tract to look as broad as possible.
Then freeze the image, and scroll back to measure the diameter of the valve hinge points at end systole.
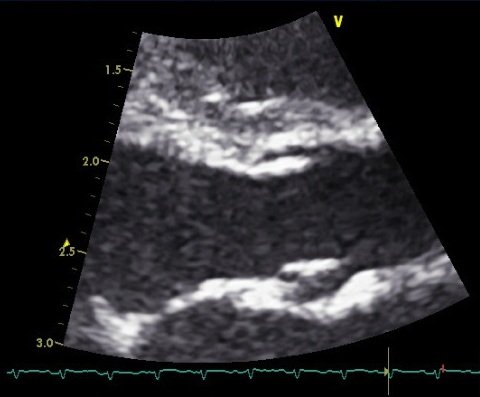
M mode measurement is not recommended as the aortic valve moves downwards during systole, and accurate placement of the M mode plane is essentially impossible.
For flow velocity use a modified apical 4 chamber view. As much as possible slide the transducer to the subject’s left, and caudally, before rotating clockwise and tilting to look anterior to the subjects right shoulder (this is the 'five chamber view').
Always use pulsed wave Doppler to prevent contamination from other sites of flow
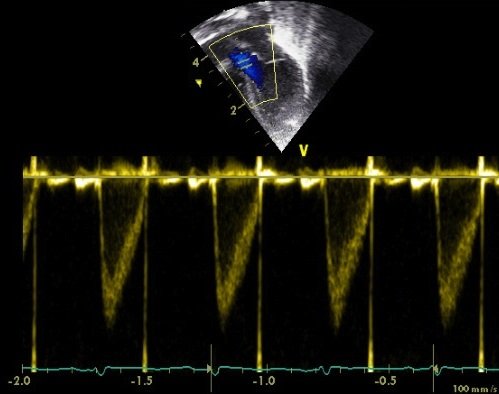
This measurement is also relatively well validated, though the valve diameter measurement is vulnerable to error since it relies on lateral rather than axial resolution of the ultrasound beam. In the newborn infant it is confounded by inter-atrial shunting, however since this is generally lower volume than ductal shunting it may actually be a more robust measure of systemic perfusion than left ventricular output in the early newborn period.
Measure the pulmonary valve diameter from the modified parasternal long axis view, with tilting to look over the subject’s left shoulder.
Zoom in on the valve, and try to get the outflow tract to look as broad as possible. Then freeze the image, and scroll back to measure the diameter of the valve hinge points at end systole.
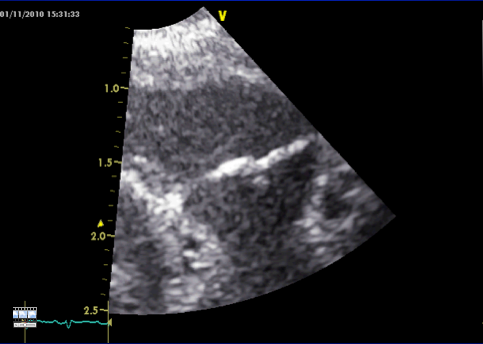
For flow velocity use the same view, with pulsed wave Doppler at the valve hinge points
Since left ventricular output includes blood about to pass through the PDA and right ventricular output includes blood which has already passed through the patent foramen ovale neither measure reflects true systemic blood flow. To circumvent this problem Nick Evans and Martin Kluckow realised the potential of measuring the volume of superior vena caval flow as a marker of upper body blood flow.
To measure the diameter of the vessel use an adapted parasternal long axis view - slide the probe superiorly and rotate slightly clockwise - essentially the SVC diameter view is a hybrid between the parasternal long axis view and the ductal view. Identify the ascending aorta then tilt the probe to look slightly to the subject’s right and the SVC should appear.
However most would agree that this view is hard to obtain. The appearance of spontaneous contrast can make the SVC distinctive. Be aware that the right atrial appendage wraps around the posterior aspect of the SVC, and can be mistaken for the SVC, especially on the M mode view.
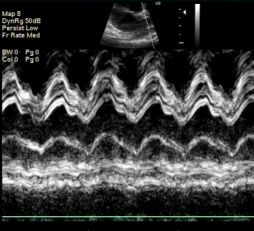
Some of the difficulty in obtaining a reliable SVC diameter measurement may be due to the unusual shape of the SVC. In cross-section it appears oval, or even crescent-shaped as it adheres to the posterior wall of the ascending aorta:
Whether SVC flow volume can be more accurately assessed by using this axial view is under study, but initial results appear promising - see Ficial et al.
For flow velocity classically a low subcostal view between the xiphisternum and the umbilicus is used. Tilt to look anterior, and slide as low as possible until you can see a clear length of the SVC flowing into the right atrium.
Flow can be measured by pulse wave Doppler. Most experts recommend taking a mean of 10 cycles due to the significant variation with respiration:
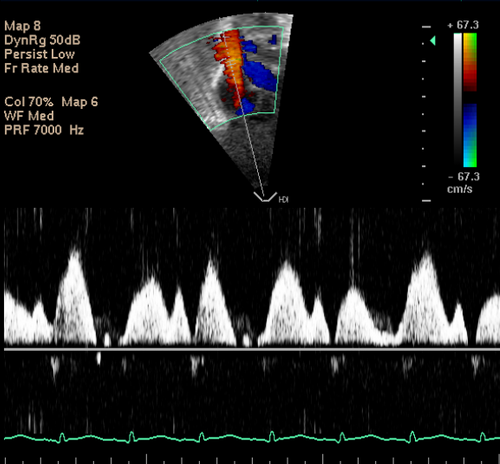
Flow velocity may also be measured from a suprasternal view, as originally described by Harabor et al. It may be that this method gives more robust measures, since it may be less afected by respiratory motion, and imaging further away from the right atrium may be a site with less turbulence, however again further study is required to fully assess the methodology.
SVC Flow Imaged Suprasternally from Neonatalechoskills.com on Vimeo.
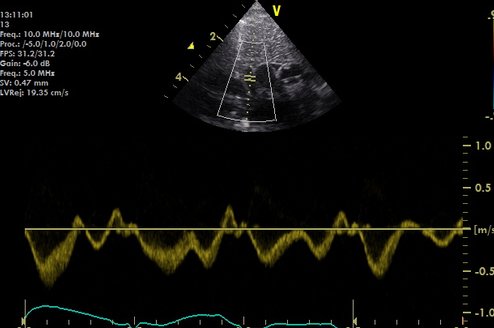
This measure is only currently recommended for use as a research tool as it has poor reproducibility! Measure the descending aortic diameter (or area) from an axial view at the level of the diaphragm.
Use high resolution zoom to optimise the DAo view:
Measure the descending aortic flow velocity from the ductal view, but with the transducer directed caudally to visualise the vessel anterior and to the left of the spine.
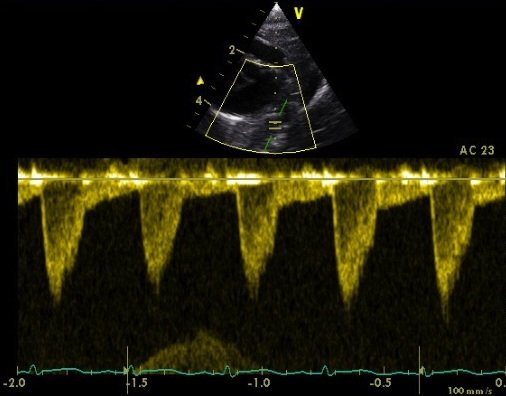
Due to angle of insonation angle correction must be used to avoid significantly underestimating volume of flow. This view is the same as used when looking for diastolic descending aortic flow reversal as part of an assessment of haemodynamic significance of the ductus arteriosus.
This is one of the most basic measures in adult functional echocardiography. It simply looks at the degree of shortening of the left ventricular diameter between end-diastole and end-systole.
To make the measure use the parasternal long axis view. Place the M mode cursor at the level of the mitral valve tips (so you can see the leaflets flicking in and out of view through the cardiac cycle).
Once the view is obtained measure the end-diastolic diameter (EDD) and end-systolic diameter (ESD). FS = (EDD-ESD)/EDD
As ever this method has some limitations. The left ventricle is often not circular in the newborn period (due to relatively high right ventricular pressure) so assumptions about geometry are not satisfied. In addition it is hard to specify the exact location of the mitral valve tips, and imaging slightly closer to the apex or base of the left ventricle is likely to raise or lower the fractional shortening respectively.
This is potentially one of the most valuable uses for functional echocardiography in the NNU, particularly to guide aggressiveness of fluid resuscitation in the collapsed neonate. However the measures have not currently been standardised, so assessments of filling volume are subjective.
Inferior Vena Caval Filling
To assess IVC filling place the ultrasound transducer in the midline, just below the xiphisternum, and in the sagittal plane. The probe marker should be pointing upward, so that the heart appears just visible on the right of the screen. The IVC can be seen coursing through the liver.
A normally filled IVC will have some pulsation with the cardiac cycle and respiratory motion. An under-filled IVC will be barely visible, or collapse entirely on inspiration:
An over-filled IVC will appear large, and minimally pulsatile. BUT BEWARE the child on the ventilator, especially high frequency oscillatory ventilation - high intrathoracic pressure can effectively tamponade venous return at the level of the IVC, making the IVC appear well-filled, when the cardiac chambers themselves are under-filled.
Therefore when assessing preload status always examine the intra-cardiac filling too.
Intra-cardiac Filling
This is often most easily accomplished from the sub-costal view, using the view used to interrogate the atria. This is convenient as it can follow directly on from the sagittal subcostal view used for IVC assessment.
Tissue Doppler uses essentially the same methodology as standard Doppler which looks at blood flow, except that rather than measuring the high velocity low signal traces from the blood pool it measures low velocity high signal traces from the myocardium. At present the techniques are a research tool, but awareness of normative data in the newborn population is increasing.
Basic tissue Doppler measures calculate the simple velocity of myocardial motion.
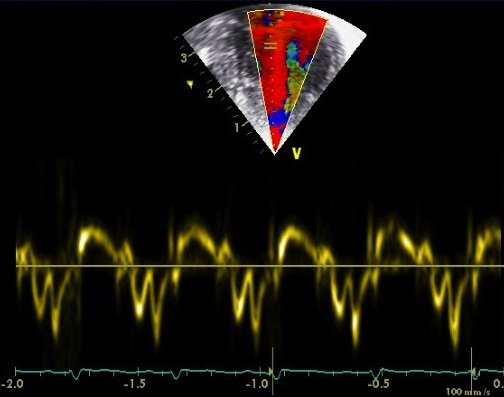
However by measuring velocity at two adjacent points in the myocardium the relative velocity between the two can be used to assess strain rate (rate of shortening) in a portion of the myocardium. By integrating strain rate against time the % strain can also be calculated.
These techniques are sensitive to image quality, artefact and angle of insonation, but they provide significant scope for the future.
This is another group of advanced imaging techniques, again at the time of writing they are a research rather than a clinical tool. While the signal received from an ultrasound scan of a thickness of myocardium may appear to be a random series of speckles, in fact the signal is generated by overlapping sheaths of fibres. Therefore signal is not randomly generated, rather it represents a ‘fingerprint’ of myocardium. Advanced post-processing techniques can now be used to track these speckles through the cardiac cycle, and again measure strain and strain rate.
These techniques are not vulnerable to angle of insonation, but do still require high quality images for processing.